america is so fucking based man
in any proper country that company at least gets forced to pay by the government then ordered to shut down forever due to wanton cruelty. all the employees get generous severance except whoever made that call. depending upon your view of carceral punishment there are a few ways to go with that guy.


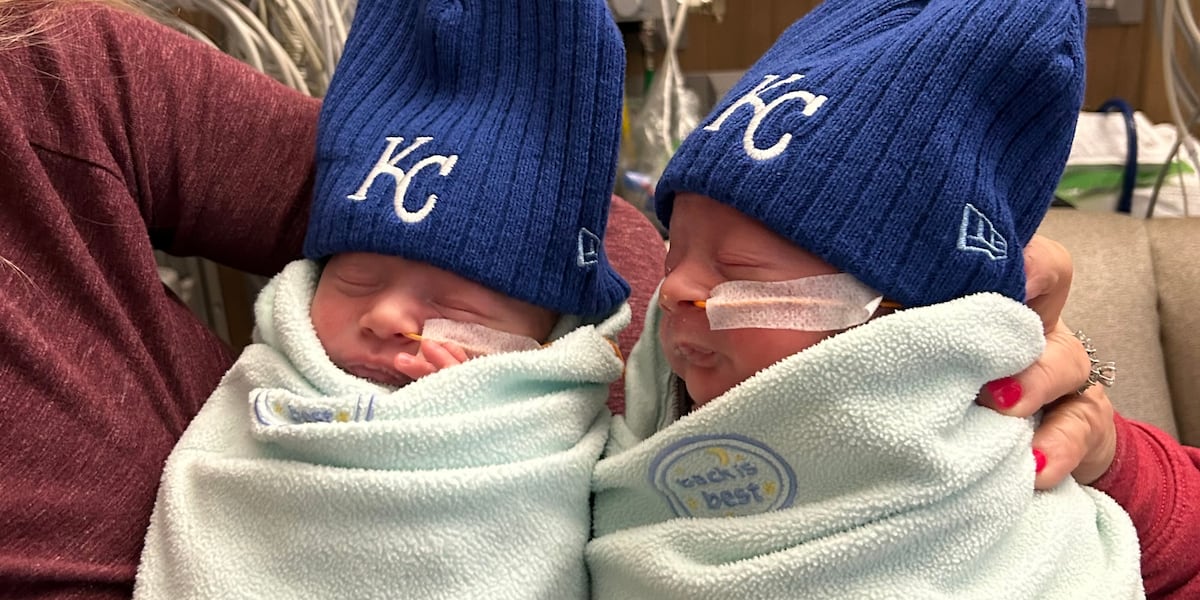
Why does the medicine cost 2.1 million in the first place? Is it just price inflation or it’s made of antimatter or something?
From https://www.drugs.com/medical-answers/zolgensma-expensive-3552644/
So there is no tangible reason why it’s this expensive.
“Because we said so and if you don’t want your kid to be a fucked up cripple loser you’ll fucking pay us, bitch.”
I have no fucking clue about this particular medicine, and Americans are getting an absolutely raw deal on healthcare
BUT
Non-greedy reasons that can raise the price of a drug:
manufacturing costs. Maybe the ingredients are expensive. Maybe the synthesis has a very low yield. Maybe storage is very expensive. Maybe storage is such a bitch the drug needs to be made on-demand. Maybe storage is straight-up impossible so the drug needs to be made on-site.
Low demand. If very few people need the drug, you can’t spread out the cost of R&D or manufacture. Furthermore, it’ll force you to use low-volume manufacturing methods, which will be more expensive. It might be so low volume that you literally just pay a chemist to synthesize the drug on a bench top, which could take weeks of labor, depending on the synthesis.
delivery mechanism. Suppose the drug itself is relatively cheap, but it needs to be delivered by a long-term release capsule implanted in your spleen. Suppose it needs to be delivered by IV drip continuously for a week. Suppose it needs to be taken under direct supervision for some reason.
Probably other shit, it’s been a while since I studied where healthcare costs come from.
Edit: lol, sounds like the justification the pharma company is going with is “fuck you, is not a child’s life worth everything you’ve got?”
You mean the part where we make sure medicine is actually medicine.
Yeah, don’t assume they were saying that’s unnecessary, just that it is expensive.
I mean the part where we make sure the medicine that can potentially save your otherwise doomed life doesn’t give you a mild rash.
Historically, large-scale withdrawals of drugs from markets ONLY occur, and large-scale marketing ONLY is barred when the side effects are deemed dangerous enough to not risk at any significant percentage. If you look through the list of withdrawn drugs throughout the world, almost all of them are withdrawn for either abuse reasons, or significant side effects like organ toxicity, serious risk of overdose even inside prescriber control, carcinogenicity, or neurological reactions (like some fungicides/bactericides causing blindness/deafness even when used properly).
SOME of these have been returned to market (like thalidomide) under very strict guidelines, used for very strict reasons (thalidomide is used for leprosy and multiple myeloma treatment now in certain situations, in combination with certain drugs to help reduce teratogenicity). Others, which were formerly seen as helpful, have been removed from markets because of newly-found dangers involving them (like Zantac, which was found to spontaneously break down into a carcinogenic compound).
Zantac? No shit? Well I’m sure glad all those zantac commercials I used to see didn’t work on me!
This is not about the cost of withdrawing or barring drugs as much as it is about the cost of running all these tests and trials. And yes, drugs can potentially have terrible side effects, but not being able to afford the drug can also have terrible results.
If the FDA requirements were much less strict, the drug company would have had to spend much less on R&D. That, of course, would not be enough to lower the price - but the other effect of cheaper R&D is that it’s easier for other companies to compete, and competition does drive prices down. The the point either the mother could either afford it herself, or the insurance wouldn’t be so stingy about paying it.
Now, of course, less strict requirements also mean we know less about the drug’s safety and efficiency. Let’s say that, because of the lack of knowledge, we assign a 50% probability for the drug to kill the patient and even if it doesn’t we only assign 50% probability for it to work (that does not mean it killed half the test subjects and failed for half of the remaining ones - just that we didn’t test enough to get significant results that say otherwise, and these are the worst case estimates under our lack of data). That means, that there is only 25% chance for each one of these twin babies to survive if they take the drug.
Which is better than the 0% they get now, being unable to afford it.
Definitely not because Novartis is trying to recoup the 8.7 billion they spent on AveXis to acquire Zolgensma…
And denying it to people who desperately need it but don’t have that kind of money is helping them recoup the costs how?
Novartis isn’t the company who are denying it, that would be the insurance company Mosaic’s Health Care Trustees who aren’t covering it because it’s too expensive as stated in the article.
Insurance companies will do anything possible to get out of paying for specialty tier medications from using ‘step therapy’ where before they will approve the medication your doctor approved you must first try and fail all the available medications starting from least expensive. Insurance companies typically also charge a presentage on speciality drugs for the copay as a deterrent from being prescribed those medications. Currently the advocacy side of some large disease-focused charities such as the arthritis foundation are trying to get congress to federally ban step therapy and set a cap for specialty tier drug copays like they have for the other drug tiers.
Shit legislation with lack of price-negotiation through collective bargaining, is the main issue, costs land at the individual enduser in the end. They could’ve likely gotten half the money and save the twins but instead they’ll get none with that pricetag calculated from putting a number to the value of a human life - that makes most insurers pull out the fineprint and drop coverage overnight…
This talks about it
https://www.canada.ca/content/dam/hc-sc/documents/services/health-related-consultation/National-Strategy-High-Cost-Drugs-eng.pdf
But it’s not really of any value because it basically says “we don’t know” and it’s not only Canada