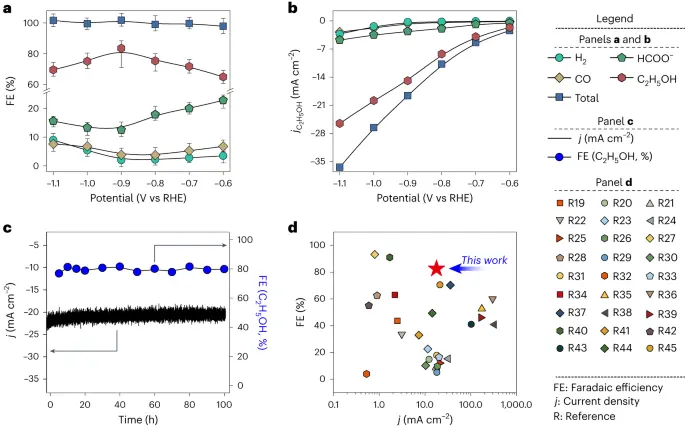
Some Chinese researchers have found a new catalyst for electrochemically reducing CO2. Multiple such catalysts are known, but so far, only copper favours reaction products with a carbon chain of at least 2 carbons (e.g. ethanol).
The new catalyst requires a specific arrangement of tin atoms on tin disulphate substrate, seems to work in a solution of potassium hydrogen carbonate (read: low temperature) and is 80% specific to producing ethanol - a very practical chemical feedstock and fuel.
The new catalyst seems stable enough (97% activity after 100 hours). Reaction rates that I can interpret into “good” or “bad” aren’t found - it could be slow to work. The original is paywalled, a more detailed article can be found at:
Carbon-Carbon Coupling on a Metal Non-metal Catalytic Pair
Overall, it’s nice to see some research into breaking down CO2 for energy storage, but there is nothing practical (industrial) on that front yet, only lab work.